The Story of Idelalisib
This blog discusses the story of Idelalisib, a drug approved by the FDAOncology for CLL and FL between 2008 and 2014. It examines the safety signal for the drug in 2016, the millions earned from it, and the horrible failure of the FDAOncology.

Vinay Prasad MD MPH
Professor @ucsf, Physician-Scientist, Writer; More at @vkprasadlab @plenary_session, YouTube, #vpzd podcast & @Sensible__Med; Views are mine
-
Our LATEST in @JAMAInternalMed
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
FDA was aware of a SAFETY signal for Idelalisib in 2016. The drug remained on market till 2023!
Meanwhile trials failed & were halted, millions earned, & safety signal mounted.
Horrible @FDAOncology failure 👇
See 🧵https://t.co/PxNH7Y4O2t pic.twitter.com/M7bhHVyxwl -
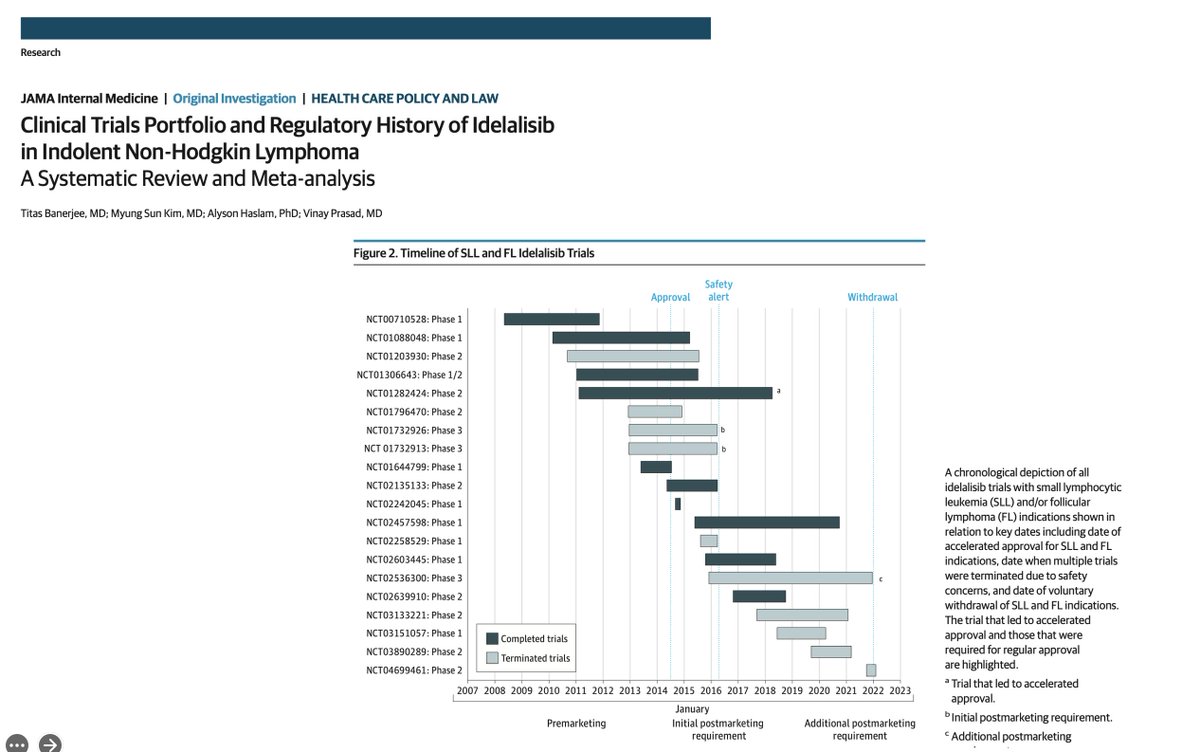
The story of idelalisib began btw 2008 and 2014 when multiple, (mostly) uncontrolled trials led the @FDAOncology to approve the drug for CLL and FL based on shrinking tumors on CT scans and not living longer or better pic.twitter.com/s16GNzuGUI
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023 -
Then in the years that followed, multiple trials had safety concerns, leading to a warning. pic.twitter.com/anG5BLgZCK
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023 -
In fact pic.twitter.com/qahH0bt9Eu
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023 -
Yet, @FDAOncology allowed the product to remain on market till "voluntary" withdrawal in 2022
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
During this time many more trials were launched pic.twitter.com/s2p6nzYydu -
Here is what is concerning. This is a cumulative meta-analysis of harm
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
Remember FDA did not take stronger action in 2016
Look at Serious adverse events rise and look at sales in millions pic.twitter.com/eNRYUY4byY -
Look at fatal adverse events pic.twitter.com/AUguKpi3WI
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023 -
Now look at deaths
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
The confidence interval is narrowing, but it is not on the good side pic.twitter.com/lLs1v34Ihx -
We describe 5 key findings
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
1. Less than half of studies are RCTs
2. Most trials remain UNPUBLISHED
3. Phase 3 data available were consistently CONCERNING
4. Product was withdrawn after addition 6! years on US maker
5. Company kept earning pic.twitter.com/XU6ojtLrNk -
Could the product have been withdrawn sooner? pic.twitter.com/8DGLbleNFE
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023 -
Also why did the FDA continue to approve multiple next in class drugs??
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
we discuss elsewherehttps://t.co/iZS5hnIgsm pic.twitter.com/mBHWDEbuwj -
This project was funded by @Arnold_Ventures
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
All credit should go to Titas Banerjee, MD
We actually had a plaque made for her for just how much effort this took. I will update this thread with a picture of that plaque -
Everyone should read the paper because this is about an FDA who is hungry to approve products, but has no appetite to regulate after approval
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
It consistently delays post market studies, and fails to take safety seriouslyhttps://t.co/PxNH7Y4O2t -
Pls draw attention to this important paper and issue
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
And remember FDA has not made Pfizer complete the post marketing myocarditis study in young men to date.
How is that acceptable?https://t.co/PxNH7Y4O2t -
Follow @vkprasadlab and https://t.co/G8QbwPGGW4 for updates
— Vinay Prasad MD MPH (@VPrasadMDMPH) March 26, 2023
