PaxLC Clinical Trial for Long COVID Patients Now Open
A phase 2, 1:1 randomized, double-blind, placebo-controlled research study in 100 non-hospitalized highly symptomatic long COVID patients is now open. The primary outcome will be measured by asking the patients to fill out some questionnaires pre & post treatment.

Prof. Akiko Iwasaki
We study innate and adaptive immunity against viruses and study disease pathogenesis. #COVID19 #longCOVID #vaccines @HHMINEWS @YaleIBIO @YaleMed @YaleCII
-
Very excited that our PaxLC clinical trial on #longCOVID patients is now open, led by @hmkyale! This is a phase 2, 1:1 randomized, double-blind, placebo-controlled research study in 100 non-hospitalized highly symptomatic long COVID. (1/)https://t.co/hga9nt8EWv
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
We will be providing paxlovid or placebo pills in long haulers for 15 days. The primary outcome will be measured by asking the patients to fill out some questionnaires pre & post treatment. We will find out whether their health changes with paxlovid vs. placebo. (2/)
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
Before, during, and after treatment, we will do a deep dive immune profiling to see if any changes are detected due to Paxlovid. We will apply a similar strategy that we used in our study with @PutrinoLab to understand the immune and viral signatures (3/)https://t.co/8cGj51HyGn
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
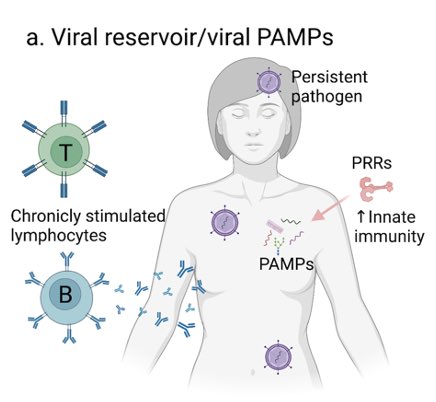
We suspect that a subset of #longCOVID patients have viral reservoirs. The reason we use a 15-day trx (not a 5-day used for acute infection) is to allow the drug to eliminate the virus that is causing chronic infection and to remove such reservoirs. (4/)https://t.co/eLuuCOaWCG pic.twitter.com/etfOP9Y4ai
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
A large number of publications demonstrate viral antigens and/or RNA in multiple organs months after the initial SARS-CoV-2 infection. We don’t know how frequently this happens and whether this causes long COVID, but our trial will tell us if there’s any link... (5/)
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
…between replicating virus and symptoms, and whether paxlovid makes a difference. So even if only a handful of long haulers benefit, we can find biomarkers of responsiveness and classification for this endotype of LC. (6/)
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
It is also possible that viral RNA and antigens seen months post-COVID in various organs do not represent replication-competent viruses. A great review by Prof. Diane Griffin summarizes how viral RNA can persist for years after acute infection. (7/)https://t.co/qJtbhm3qu6
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
If viral RNA persists in the absence of replication, Paxlovid will not be able to get rid of it. We will need alternative approaches. Even if we can’t get rid of vRNA, we can interfere with the downstream inflammatory consequences of vRNA by blocking host signals. (8/)
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
I want to also highlight similar trials to test Paxlovid in people with long COVID. Collectively, we will learn a lot from these trials and improve upon future approaches. (9/)https://t.co/SaBW07851yhttps://t.co/zuphOsen2C
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023 -
We are so grateful and excited to get started. The study will recruit participants through the Yale LISTEN study, and conducted at @YaleCII and @YaleCORE. More information about the enrollment will be coming online soon. https://t.co/8qi3ZhYruy (end)
— Prof. Akiko Iwasaki (@VirusesImmunity) March 21, 2023
